AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2024-11-05 View volume: 307
Case 1:
1. Expression system optimization: host strains, induction temperature, induction time, inducer concentration, and culture media.
| Host Strains | Induction Temperature | Induction Time | Inducer | Culture Media |
|---|---|---|---|---|
| T7E, BL21, C41, Arctic | 16°C/37°C | 16h/4h | IPTG | LB/Auto-induction medium |
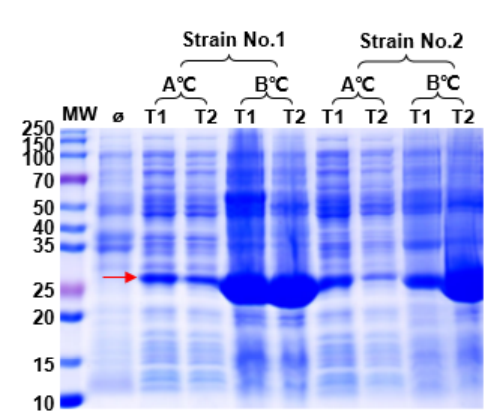
Fig. 1: Optimization of Protein Expression Conditions
2. 3C enzyme was used to remove the His-tag from the target protein. The results of enzyme digestion and protein QC are shown in Fig. 2 and Fig. 3.
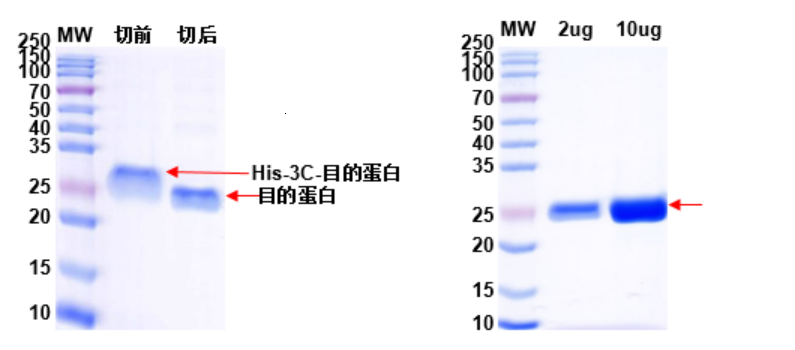
Fig. 2: Removal of Target Protein His-tag with 3C Enzyme Fig. 3: QC Analysis of His-tag Removed Protein
Case 2:
Antibody fragments were expressed in E. coli. Due to the presence of disulfide bonds, incorrect folding during expression led to inclusion bodies. By targeting the periplasmic space for expression, properly folded soluble antibody fragments containing disulfide bonds were obtained.
1. Protein expression and QC results are shown in Fig. 4 and Fig. 5.
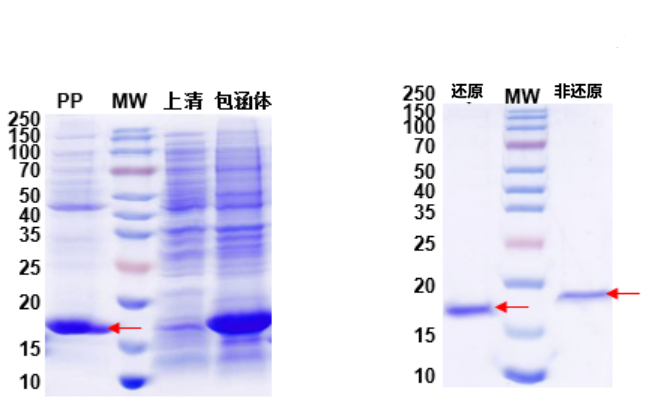
Fig. 4: Periplasmic Expression of Antibody Fragment Fig. 5: Reduced and Non-reduced QC of Antibody Fragment
Lane MW: Protein marker. Lane PP: Periplasmic Space
Case 3:
Many proteins expressed in E. coli form inclusion bodies due to the host's limitations. To ensure the functionality of the target protein, inclusion bodies are refolded into correctly folded soluble proteins for activity assays.
1. Protein expression and QC results are shown in Fig. 6 and Fig. 7.
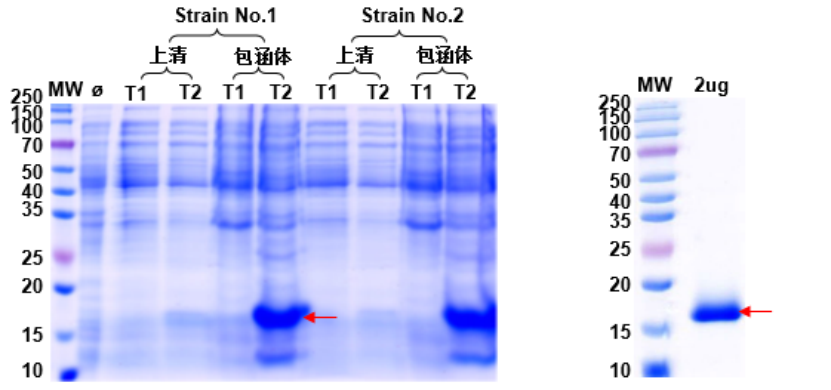
Fig. 6: Optimization of Target Protein Expression Fig. 7: Refolding QC of Target Protein
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

